
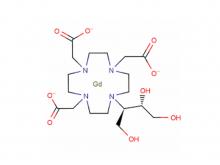
Recently, Beijing Beilu Pharmaceutical Co., Ltd. (Beilu Pharma) obtained the Drug Registration Certificate for Iomeprol Injection, officially approved and issued by the National Medical Products Administration (NMPA) of China.
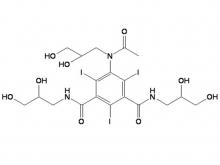
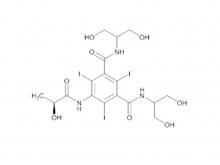
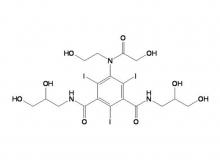
Iomeprol Injection, developed by Bracco Group in Italy, is a non-ionic monomeric X-ray contrast agent. Boasting excellent physicochemical properties, it features the lowest osmotic pressure and lower viscosity at the same concentration compared to other non-ionic monomeric X-ray contrast agents of the same class. Its stable physicochemical properties also eliminate the need for chelating agents. Indications include: intravenous urography (adults, including those with renal impairment or diabetes), CT(Trunk), routine angiography, arterial DSA, cardiovascular angiography (adults and pediatric patients), routine selective coronary angiography, interventional coronary angiography, fistulography, ductography, dacryocystorhinostomy, and sialocystography.
The product has been included in China’s National Medical Insurance Category B List. Data from Menet shows that its sales across China’s three major terminals and six key markets have maintained double-digit growth in recent years,: exceeding RMB 1.1 billion (approx. USD 152 million) in 2024, representing a year-on-year increase of approximately~32%; and exceeding RMB 300 million (approx. USD 41.5 million) in Q1 2025, with a year-on-year growth of approximately~39%.
Approved as a Class 4 Chemical Drug, Iomeprol Injection is deemed to have passed the the consistency evaluation of generic drug quality and efficacy. This approval further enriches Beilu Pharma’s iodine contrast agent portfolio, enhancing the diversification of its contrast agent product line. Zhejiang Hichi Pharmaceutical Co., Ltd., a holding subsidiary of Beilu Pharmaceutical, secured market approval for Iomeprol Active Pharmaceutical Ingredient (API) in August 2025, solidifying the "API + Formulation" integrated business model for the product. Beilu Pharma will actively advance preparations for the new products launch and bring them to market as soon as possible.
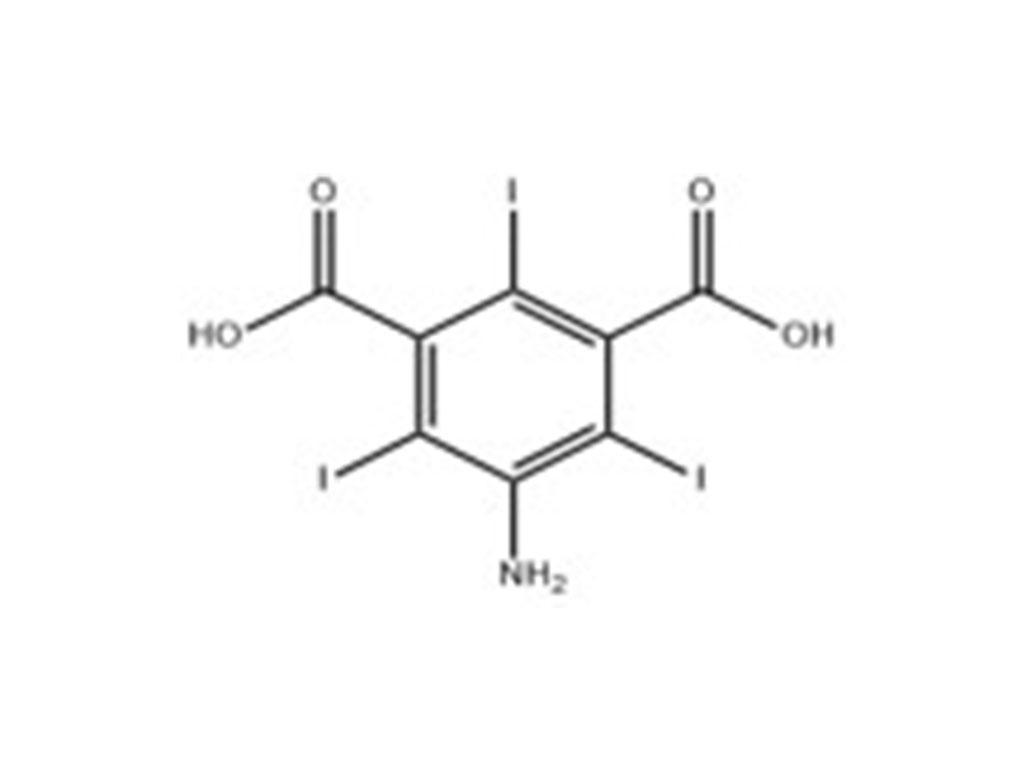
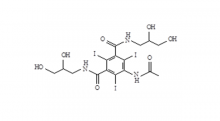 Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
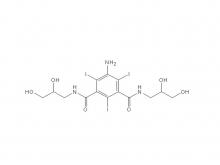 Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
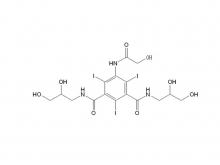 Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
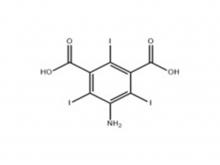 Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
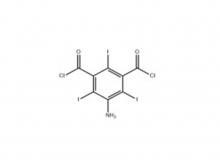 Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
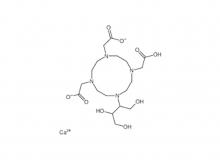
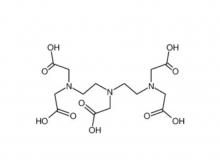 Diethylenetriaminepentaacetic acid (DTPA)
Diethylenetriaminepentaacetic acid (DTPA)

 EN
EN
 jp
jp  fr
fr  de
de  es
es  ru
ru  ar
ar 










 Call us on:
Call us on:  Email Us:
Email Us:  No.3 Shuiyuan West Road, Miyun District, Beijing, China
No.3 Shuiyuan West Road, Miyun District, Beijing, China 