The quality establishment is the core content of Beijing Beilu Pharmaceutical enterprise culture. The company always puts the quality in the first place, so the complete quality control system was built, the materials, media, intermediate products, products to be packaged and finished products under well control each level and the whole production process, storage process, transportation process and sales process under whole monitored synchronously.
-
Home
-
Products
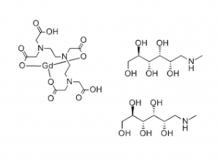
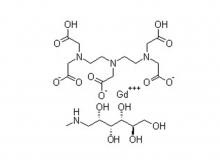
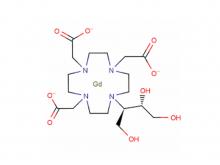
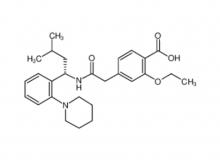
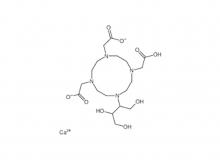
- Gadopentetate Dimeglumine Injection/API Gadobutrol Injection/API Gadobenate Dimeglumine Injection Ferric Ammonium Citrate Effervescent Granules
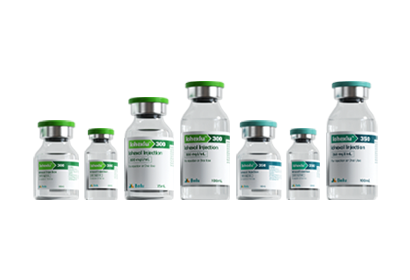
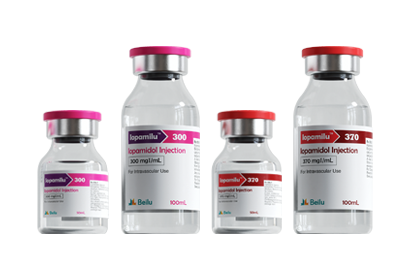
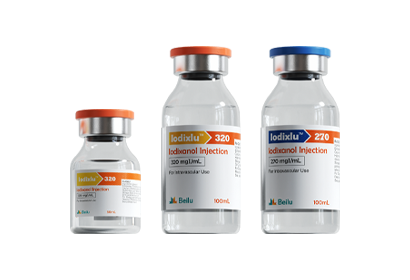
- Iohexol Injection Iopamidol Injection Iodixanol Injection
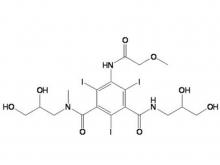
- Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
- Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
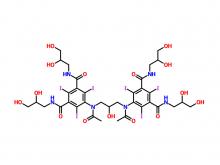
- Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
- Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
- Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
- Diethylenetriaminepentaacetic acid (DTPA)
-
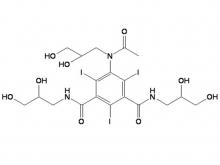
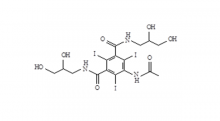 Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
-
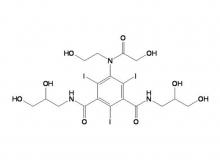
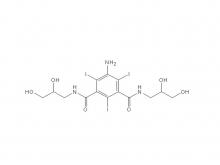 Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
-
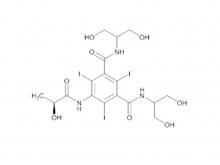
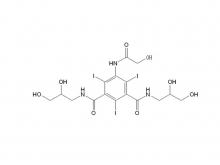 Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
-
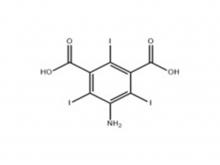 Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
-
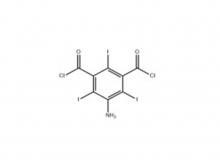 Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
-
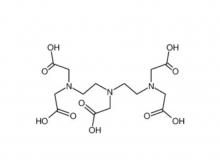 Diethylenetriaminepentaacetic acid (DTPA)
Diethylenetriaminepentaacetic acid (DTPA)
- Company
- News
- Contact Us

 EN
EN
 jp
jp  fr
fr  de
de  es
es  ru
ru  ar
ar 




.jpg)






 Call us on:
Call us on:  Email Us:
Email Us:  No.3 Shuiyuan West Road, Miyun District, Beijing, China
No.3 Shuiyuan West Road, Miyun District, Beijing, China 