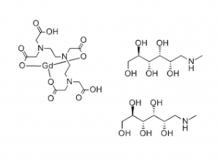
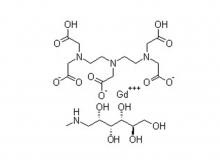
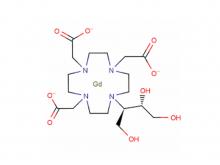
Safety of Gadolinium Contrast Media
Acute reactions
Gadolinium Contrast Media possess a very low incidence ( < 2.5 % ) of acute adverse reactions compared to many other drugs. Almost all of their reactions are very mild, the most common are nausea, headache, discomfort at the injection site, vomiting, metallic taste, paresthesia, fever and dizziness.
Immediate hypersensitivity reactions may occur within 1 hour of imaging agents administration, it is approximately 1 in 1000 cases. These are more likely to occur in patients with allergies, asthma, and allergic reactions to gadolinium contrast agents. The most common of these reactions are mild pruritus ( itching ) and urticarial ( hives ). The moderate hypersensitivity reactions occur in about 1 in 5000 cases, it usually includes bronchospasm, facial oedema, arrhythmias, laryngospasm, tachycardia, or widespread urticarial(hives).
The incidence of severe reactions is about 1 in 20,000. A number of severe anaphylactoid reactions to Gd MRI Contrast Media, including death, have been reported worldwide, the risk of death is about 1 in 400,000.
Chronic Reactions
NSF ( Nephrogenic Systemic Fibrosis ) is the most famous of Chronic reactions to Ga Contrast Media. It is a disease of fibrosis of the skin and internal organs, which occurs in patients with chronic severe renal insufficiency. Several hundred patients have been diagnosed worldwide since the first reported case in 2006. NSF has now been largely eliminated as an existing disease with the efforts of the biology community. However, Gd-containing plaques in the extremities have been reported and may represent a forme fruste of NSF.
The US FDA believes that although medical literature reports that there is the deposition of gadolinium-containing Contrast Media in the brain tissue of some patients undergoing 4 or more MRI scans, so far, there are no harmful symptoms, signs, and pathological changes appearing associated with the gadolinium-based contrast agent deposition in brain tissue. In its database of adverse event reporting system, the FDA has received many adverse event reports of patients experiencing pain or other symptoms after single or multiple applications of gadolinium-containing Contrast Media. However, so far, the FDA hasn't been able to clarify common features in these reports. It is not proven yet that the reported symptoms are reasonably related to gadolinium. Further information is needed to evaluate the possible risks caused by gadolinium deposition.
In conclusion, although the incidence of adverse reactions of the gadolinium Contrast Media used in the MRI enhancement examination is very low, one should always keep in mind that any drug administration carries with it the risk of life-threatening reaction, and long-term risks of gadolinium contrast medium deposition must be considered.
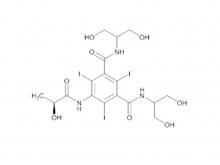
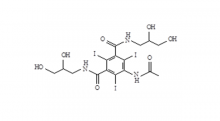 Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
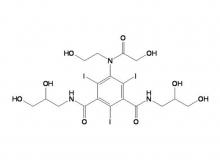
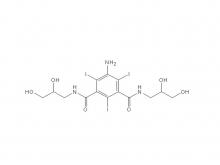 Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
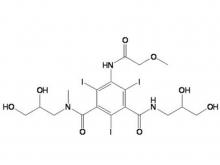
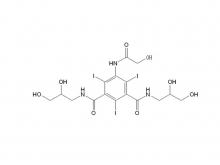 Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
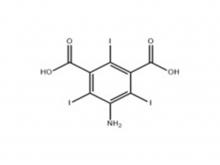 Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
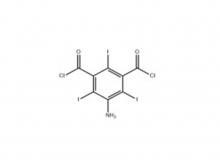 Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
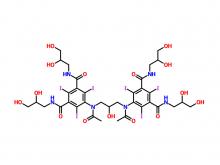
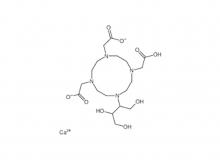
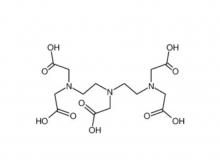 Diethylenetriaminepentaacetic acid (DTPA)
Diethylenetriaminepentaacetic acid (DTPA)

 EN
EN
 jp
jp  fr
fr  de
de  es
es  ru
ru  ar
ar 



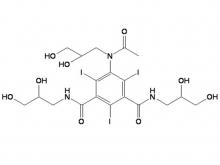



 Call us on:
Call us on:  Email Us:
Email Us:  No.3 Shuiyuan West Road, Miyun District, Beijing, China
No.3 Shuiyuan West Road, Miyun District, Beijing, China 