-
Home
-
Products
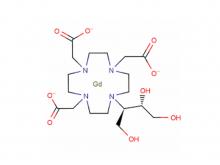
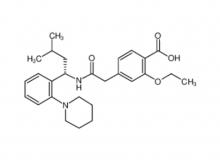
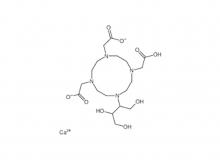
- Gadopentetate Dimeglumine Injection/API Gadobutrol Injection/API Gadobenate Dimeglumine Injection Ferric Ammonium Citrate Effervescent Granules
- Iohexol Injection Iopamidol Injection Iodixanol Injection
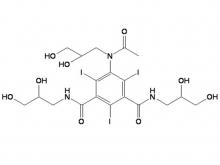
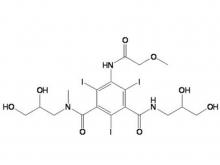
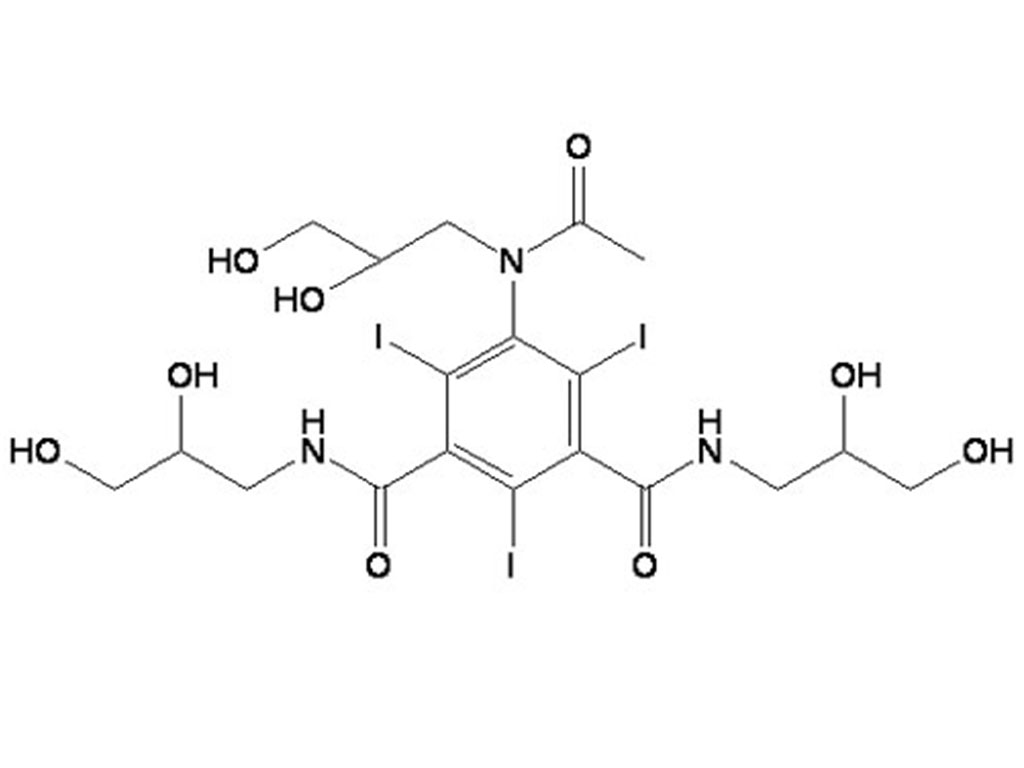
- Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
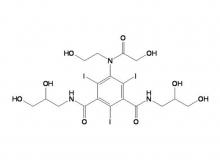
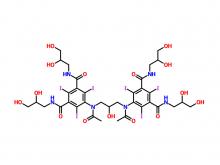
- Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
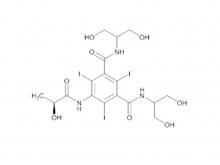
- Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
- Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
- Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
- Diethylenetriaminepentaacetic acid (DTPA)
-
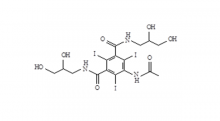 Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
-
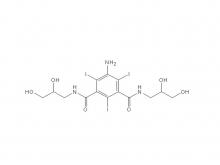 Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
-
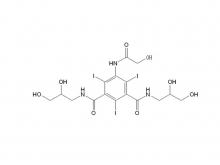 Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
-
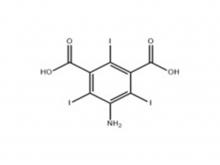 Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
-
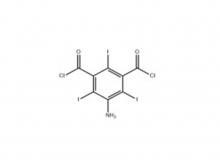 Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
-
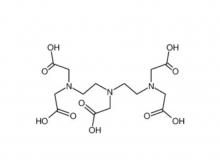 Diethylenetriaminepentaacetic acid (DTPA)
Diethylenetriaminepentaacetic acid (DTPA)
- Company
- News
- Contact Us

 EN
EN
 jp
jp  fr
fr  de
de  es
es  ru
ru  ar
ar 







 Call us on:
Call us on:  Email Us:
Email Us:  No.3 Shuiyuan West Road, Miyun District, Beijing, China
No.3 Shuiyuan West Road, Miyun District, Beijing, China 