Product name: Repaglinide Tablets
Characteristic: White or off-white tablet
Specification: repaglinide 0.5mg×30 tablets, repaglinide 0.5mg×60 tablets.
Indications:
It is used for type 2 diabetes (non-insulin-dependent) patients whose hyperglycemia cannot be effectively controlled by diet control, weight loss and exercise. When metformin alone cannot effectively control hyperglycemia, Repaglinide tablets can be combined with metformin. Treatment should start from the adjuvant treatment of diet control and exercise to reduce blood sugar during meals.
Specifications of Repaglinide Tablets: 0.5mg
Usage and Dosage,Adverse reactions,Contraindications,Precautions:
Please refer to the package insert.
Medicine Interactions:
Some drugs are known to affect glucose metabolism. Therefore, doctors should consider possible drug interactions.
In vitro studies have shown that the metabolism of Repaglinide is affected by CYP2C8 and CYP3A4. Repaglinide was found to be a substrate for active liver uptake (organic anion transporter OATP1B1).
Data from clinical studies carried out in healthy volunteers show that CYP2C8 is the main enzyme in the metabolism of Repaglinide, while CYP3A4 has limited effects. But if the effect of CYP2C8 is inhibited, the effect of CYP3A4 will be relatively enhanced. Therefore, the metabolism and clearance of Repaglinide may be changed due to the inhibition or induction of cytochrome P450. Therefore, extreme caution should be exercised when using CYP2C8 and CYP3A4 inhibitors simultaneously with Repaglinide. Drugs that inhibit OATP1B1 (such as cyclosporine) may increase the plasma concentration of Repaglinide. ( For more details, please refer to the package insert.)
Storage: Similar to the storage of glimepiride tablets, tab repaglinide should also be shaded, sealed, kept in a dry place.
Packaging:
Aluminum plastic packaging, 15 pills/plate , 2 plates/box; 15 pills/plate, 4 plates/box, 15 pills/plate, 6 plates/box
Shelf life: 24 months
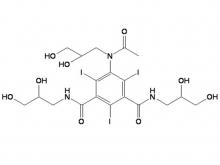
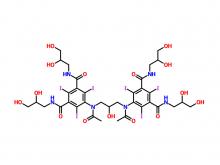
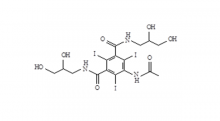 Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol Intermediate 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
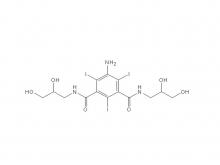 Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
Iohexol/Ioversol Intermediate 5-Amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
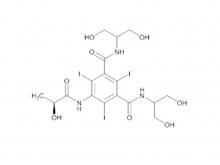
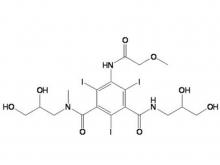
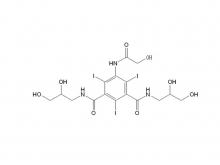 Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
Ioversol Intermediate (order based) N, N'-Bis(2,3-dihydroxypropyl)-5-(glycoloylamino)-2,4,6-triiodoisophthalamide
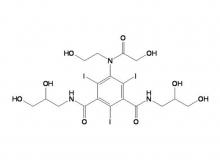
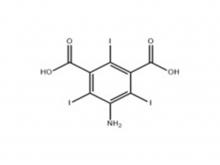 Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
Iopamidol Intermediate (order based) 5-Amino-2,4,6-triiodoisophthalic acid
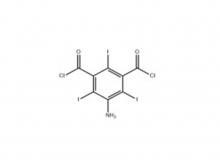 Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
Iopamidol Intermediate (order based) 5-Amino-2,4,6- triiodisophthaloyl acid dichloride
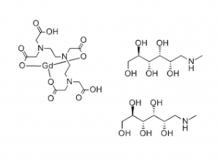
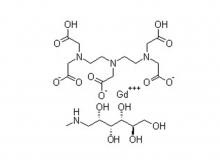
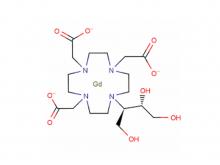
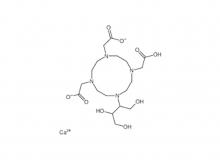
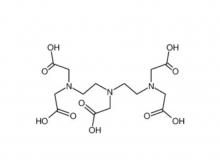 Diethylenetriaminepentaacetic acid (DTPA)
Diethylenetriaminepentaacetic acid (DTPA)

 EN
EN
 jp
jp  fr
fr  de
de  es
es  ru
ru  ar
ar 



![[News] Beilu Pharmaceutical's Wholly-owned Subsidiary, Hong Kong Yuanzhi, Obtains Hong Kong Proprietary Chinese Medicine Registration Certificate for Jiuwei Sleep-Aid Granules [News] Beilu Pharmaceutical's Wholly-owned Subsidiary, Hong Kong Yuanzhi, Obtains Hong Kong Proprietary Chinese Medicine Registration Certificate for Jiuwei Sleep-Aid Granules](https://www.beilupharma.com/themes/simple/img/news4.jpg)
 Call us on:
Call us on:  Email Us:
Email Us:  No.3 Shuiyuan West Road, Miyun District, Beijing, China
No.3 Shuiyuan West Road, Miyun District, Beijing, China 