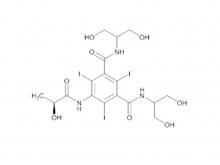
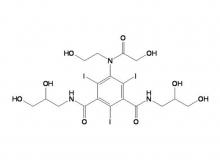
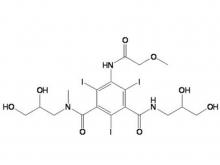
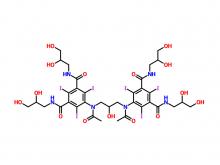
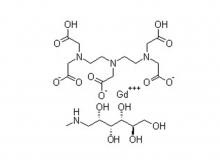
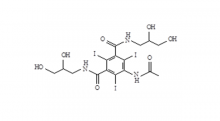
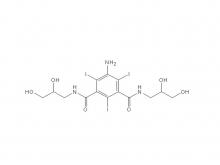
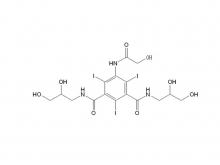
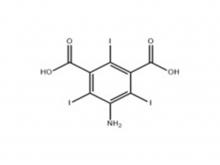
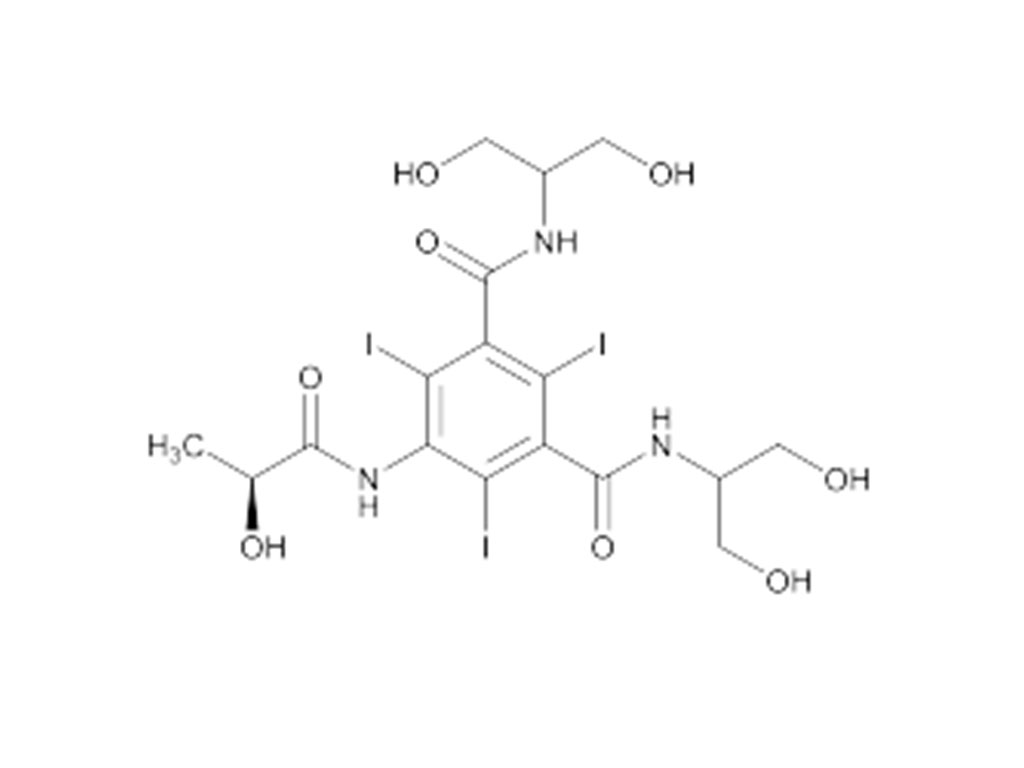
Recently, Zhejiang Hichi Pharmaceutical Co., Ltd., a subsidiary of Beijing Beilu Pharmaceutical Co., Ltd., received the Iodixanol API product registration certificate issued by the Central Drugs Standard Control Organisation (CDSCO) under the Ministry of Health & Family Welfare of India.
After successfully obtaining domestic market approval for Iodixanol API in September 2024, Hichi Pharmaceutical received the registration certificate for South Korea in March of this year, and has now obtained the registration certificate for India. This signifies that the product has successfully passed the review by the relevant Indian authorities, officially gaining market access in India. As a result, the product can now be sold in the Indian market, enhancing the company's potential to further expand its international presence. This provides strong support for the steady growth of Hichi Pharmaceutical's API sales business and helps the company enhance its competitiveness in related markets, thereby solidifying and expanding its market share.
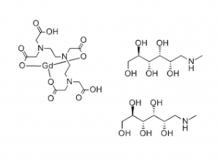
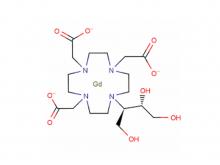
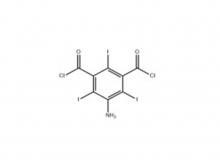
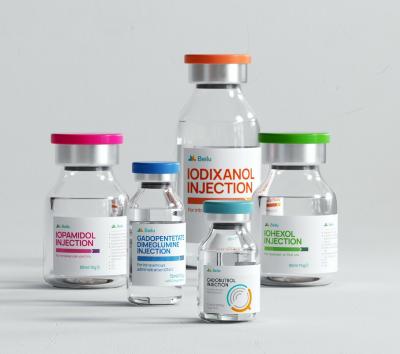
To date, Hichi Pharmaceutical has received approval for various APIs including Iohexol, Iodixanol, Iopamidol, and Iohexol, enriching its product offerings and further establishing the company's strategic layout of an integrated "API + Formulation" business for contrast agents.

 EN
EN
 jp
jp  fr
fr  de
de  es
es  ru
ru  ar
ar 








 Call us on:
Call us on:  Email Us:
Email Us:  No.3 Shuiyuan West Road, Miyun District, Beijing, China
No.3 Shuiyuan West Road, Miyun District, Beijing, China 